- Atomic Weight Calculator
- Carbon 12 Atomic Number
- Average Atomic Mass Of Carbon-12
- What Is The Atomic Mass Of Carbon 12
- The Atomic Mass Of An Element Is
| General | |
|---|---|
| Symbol | 12C |
| Names | carbon-12, C-12 |
| Protons | 6 |
| Neutrons | 6 |
| Nuclide data | |
| Natural abundance | 98.93% |
| Parent isotopes | 12N 12B |
| Isotope mass | 12 u |
| Spin | 0 |
| Excess energy | 0± 0 keV |
| Binding energy | 92161.753± 0.014 keV |
| Isotopes of carbon Complete table of nuclides | |
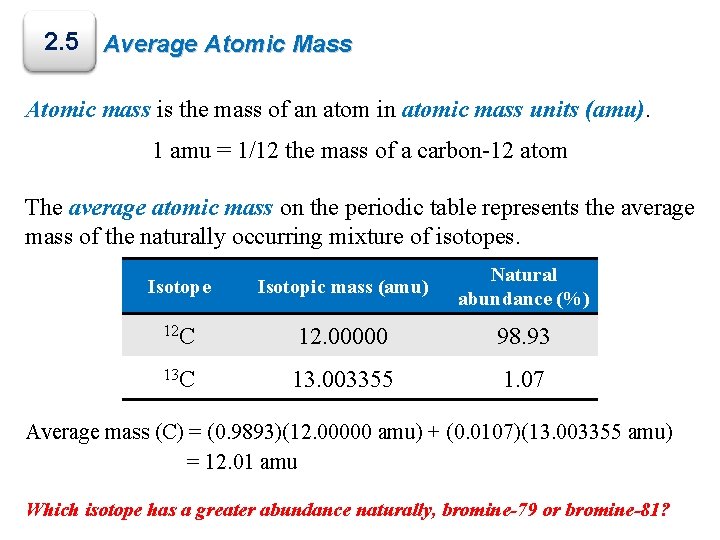
Carbon-12 (12C) is the more abundant of the two stableisotopes of carbon (carbon-13 being the other), amounting to 98.93% of the elementcarbon;[1] its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition. Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons.
12 C (12 u) and 1.07% 13 C (13.003 u). Let us assume that there are 10 000 atoms of carbon. Then you have 9893 atoms of 12 C and 107 atoms of 13 C. Mass of 12 C = 9893 atoms × 12 u / 1 atom=118 716 u. Mass of 13 C = 107 atoms × 13.003 u / 1 atom=118 716 u. Total mass= 120 110 u. Atomic mass of Carbon is 12.0107 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.
According to the image the atomic mass of carbon is 12.011, How is atomic mass of mass of carbon, or any other element, determined The atomic mass of carbon is determined by: adding the number of protons and the number of neutrons together. The carbon-12 (C-12) atom has six protons and six neutrons in its nucleus. In imprecise terms, one AMU is the average of the proton rest mass and the neutron rest mass. This is approximately 1.67377 x 10 -27 kilogram (kg), or 1.67377 x 10 -24 gram (g).
History[edit]
Before 1959, both the IUPAP and IUPAC used oxygen to define the mole; the chemists defining the mole as the number of atoms of oxygen which had mass 16 g, the physicists using a similar definition but with the oxygen-16 isotope only. The two organizations agreed in 1959/60 to define the mole as follows.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 12 gram of carbon 12; its symbol is 'mol'.
This was adopted by the CIPM (International Committee for Weights and Measures) in 1967, and in 1971, it was adopted by the 14th CGPM (General Conference on Weights and Measures).
In 1961, the isotope carbon-12 was selected to replace oxygen as the standard relative to which the atomic weights of all the other elements are measured.[2]
Atomic Weight Calculator
In 1980, the CIPM clarified the above definition, defining that the carbon-12 atoms are unbound and in their ground state.
In 2018, IUPAC specified the mole as exactly 6.022 140 76 × 1023 'elementary entities'. The number of moles in 12 grams of carbon-12 became a matter of experimental determination.
Hoyle state[edit]
The Hoyle state is an excited, spinless, resonant state of carbon-12. It is produced via the triple-alpha process, and was predicted to exist by Fred Hoyle in 1954.[3] The existence of the 7.7 MeV resonance Hoyle state is essential for the nucleosynthesis of carbon in helium-burning red giant stars, and predicts an amount of carbon production in a stellar environment which matches observations. The existence of the Hoyle state has been confirmed experimentally, but its precise properties are still being investigated.[4]
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
The Hoyle state is populated when a helium-4 nucleus fuses with a beryllium-8 nucleus in a high-temperature (108K) environment with densely concentrated (105 g/cm3) helium. This process must occur within 10−16 seconds as a consequence of the short half-life of 8Be. The Hoyle state also is a short-lived resonance with a half-life of 2.4×10−16 seconds; it primarily decays back into its three constituent alpha particles, though 0.0413(11)% of decays occur by internal conversion into the ground state of 12C.[5]
In 2011, an ab initio calculation of the low-lying states of carbon-12 found (in addition to the ground and excited spin-2 state) a resonance with all of the properties of the Hoyle state.[6][7][8]
Isotopic purification[edit]
The isotopes of carbon can be separated in the form of carbon dioxide gas by cascaded chemical exchange reactions with amine carbamate.[9]
See also[edit]
References[edit]

- ^'Table of Isotopic Masses and Natural Abundances'(PDF). 1999.
- ^'Atomic Weights and the International Committee — A Historical Review'. 2004-01-26.
- ^Hoyle, F. (1954). 'On Nuclear Reactions Occurring in Very Hot Stars. I. the Synthesis of Elements from Carbon to Nickel'. The Astrophysical Journal Supplement Series. 1: 121. Bibcode:1954ApJS....1..121H. doi:10.1086/190005. ISSN0067-0049.
- ^Chernykh, M.; Feldmeier, H.; Neff, T.; Von Neumann-Cosel, P.; Richter, A. (2007). 'Structure of the Hoyle State in C12'(PDF). Physical Review Letters. 98 (3): 032501. Bibcode:2007PhRvL..98c2501C. doi:10.1103/PhysRevLett.98.032501. PMID17358679.
- ^Alshahrani, B.; Kibédi, T.; Stuchberry, A.E.; Williams, E.; Fares, S. (2013). 'Measurement of the radiative branching ratio for the Hoyle state using cascade gamma decays'. EPJ Web of Conferences. 63: 01022–1—01022–4. doi:10.1051/epjconf/20136301022.
- ^Epelbaum, E.; Krebs, H.; Lee, D.; Meißner, U.-G. (2011). 'Ab Initio Calculation of the Hoyle State'(PDF). Physical Review Letters. 106 (19): 192501. arXiv:1101.2547. Bibcode:2011PhRvL.106s2501E. doi:10.1103/PhysRevLett.106.192501. PMID21668146.[permanent dead link]
- ^Hjorth-Jensen, M. (2011). 'Viewpoint: The carbon challenge'. Physics. 4: 38. Bibcode:2011PhyOJ...4...38H. doi:10.1103/Physics.4.38.
- ^News, Natalie Wolchover, Simons Science. 'The Hoyle State: A Primordial Nucleus behind the Elements of Life'. Scientific American. Retrieved 2020-12-06.
- ^Kenji Takeshita and Masaru Ishidaa (December 2006). 'Optimum design of multi-stage isotope separation process by exergy analysis'. ECOS 2004 - 17th International Conference on Efficiency, Costs, Optimization, Simulation, and Environmental Impact of Energy on Process Systems. 31 (15): 3097–3107. doi:10.1016/j.energy.2006.04.002.
| Lighter: carbon-11 | Carbon-12 is an isotope of carbon | Heavier: carbon-13 |
| Decay product of: boron-12, nitrogen-12 | Decay chain of carbon-12 | Decays to: stable |

Nuclear Binding Energy and the Mass Defect
A neutron has a slightly larger mass than the proton. These are often given in terms of an atomic mass unit, where one atomic mass unit (u) is defined as 1/12th the mass of a carbon-12 atom.
| Particle | Mass (kg) | Mass (u) | Mass (Mev/c2) |
|---|---|---|---|
| 1 atomic mass unit | 1.660540 x 10-27 kg | 1.000 u | 931.5 MeV/c2 |
| neutron | 1.674929 x 10-27 kg | 1.008664 u | 939.57 MeV/c2 |
| proton | 1.672623 x 10-27 kg | 1.007276 u | 938.28 MeV/c2 |
| electron | 9.109390 x 10-31 kg | 0.00054858 u | 0.511 MeV/c2 |
Carbon 12 Atomic Number
Einstein's famous equation relates energy and mass:
E = mc2
You can use that to prove that a mass of 1 u is equivalent to an energy of 931.5 MeV.
Something should strike you as strange about the table above. The carbon-12 atom has a mass of 12.000 u, and yet it contains 12 objects (6 protons and 6 neutrons) that each have a mass greater than 1.000 u, not to mention a small contribution from the 6 electrons.

This is true for all nuclei, that the mass of the nucleus is a little less than the mass of the individual neutrons, protons, and electrons. This missing mass is known as the mass defect, and represents the binding energy of the nucleus.
The binding energy is the energy you would need to put in to split the nucleus into individual protons and neutrons. To find the binding energy, add the masses of the individual protons, neutrons, and electrons, subtract the mass of the atom, and convert that mass difference to energy. For carbon-12 this gives:
Average Atomic Mass Of Carbon-12
Mass defect = Dm = 6 * 1.008664 u + 6 * 1.007276 u + 6 * 0.00054858 u - 12.000 u = 0.098931 u
What Is The Atomic Mass Of Carbon 12
The binding energy in the carbon-12 atom is therefore 0.098931 u * 931.5 MeV/u = 92.15 MeV.
The Atomic Mass Of An Element Is
In a typical nucleus the binding energy is measured in MeV, considerably larger than the few eV associated with the binding energy of electrons in the atom. Nuclear reactions involve changes in the nuclear binding energy, which is why nuclear reactions give you much more energy than chemical reactions; those involve changes in electron binding energies.
