How can we know that which element is more electronegative than other ? I've never come across any such formula or trick for this,except learning it by practice. Please tell if you know any.
Eg: in heterolytic fission of CO, we know that oxygen will take both the electrons but what if there was some other element whose relative electronegativity I dont know, then how can I solve it.
Eg: in heterolytic fission of CO, we know that oxygen will take both the electrons but what if there was some other element whose relative electronegativity I dont know, then how can I solve it.
The most electronegative atom is F, because F has short radius atom, highly energy ionization and 7 electron valence shell. Its make F easier than another atom become an ion negative 825 views. Hence, fluorine is the most electronegative of the elements (not counting noble gases), whereas caesium is the least electronegative, at least of those elements for which substantial data is available. This would lead one to believe that caesium fluoride is the compound whose. We expect polarization from the two fluorine atoms, the most electronegative atoms in the periodic table, to have a greater affect on the net dipole moment than polarization from the lone pair of electrons on nitrogen. The molecular geometry of BF3 is trigonal planar. The next halogens, which are in the next column to the left, are the most electronegative. The higher up in the row on the Periodic Table, the more electronegative. Silicon is more electronegative. Fluorine is the most electronegative element on the periodic table. Its electronegativity value is 3.98.

2 Answers
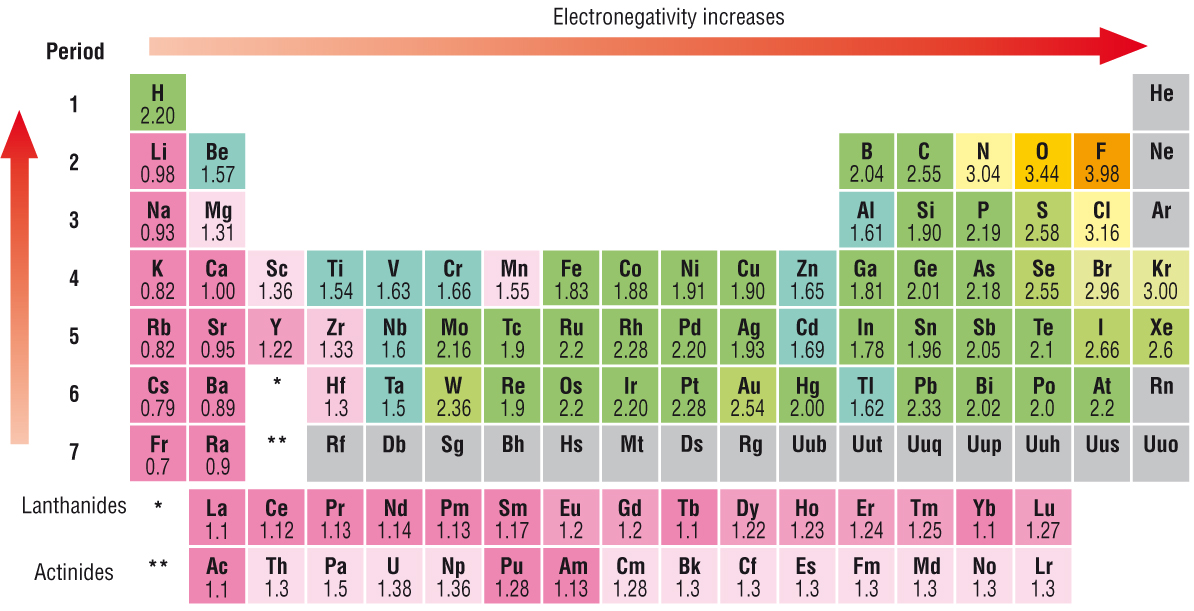
Electronegativity INCREASES across a Period, from left to right as we face the Table...........
Explanation:

And excluding the
Why should this be so? Electronegativity tends to be a function of NUCLEAR charge (and thus dependent on
Down a Group, the
And so let's have a gander at the Pauling Scale, and examine the electronegativities:
Is this representation roughly consistent with what I have argued? The most electronegative element should thus be fluorine, and indeed it is. On the other hand, the LEAST electronegative elements should be the alkali, and alkaline-earth metals. Is this true?
This is one of the most important trends a chemistry undergraduate can learn. If you are unsatisfied with this answer, or seek clarification, voice your objection, and someone will address the issue.
And thus in your example, for
Actually there are 4 or 5 formulae for calculating electronegativity according to different models.....
Explanation:
Pauling, Allen, Mullikan, Sanderson and Allred-Rochow all came up with models from which electronegativity values can be derived using formulae. None of them are especially 'simple' calculations, and you will need a decent chemical data book to hand to find the various values to plug in. However, it's certainly do-able.
Wikipedia actually lists the various methods and explains how to do them here en.wikipedia.org/wiki/Electronegativity#Methods_of_calculation
The Most Electronegative Atom In A Compound Has A Charge That Is
Related questions
