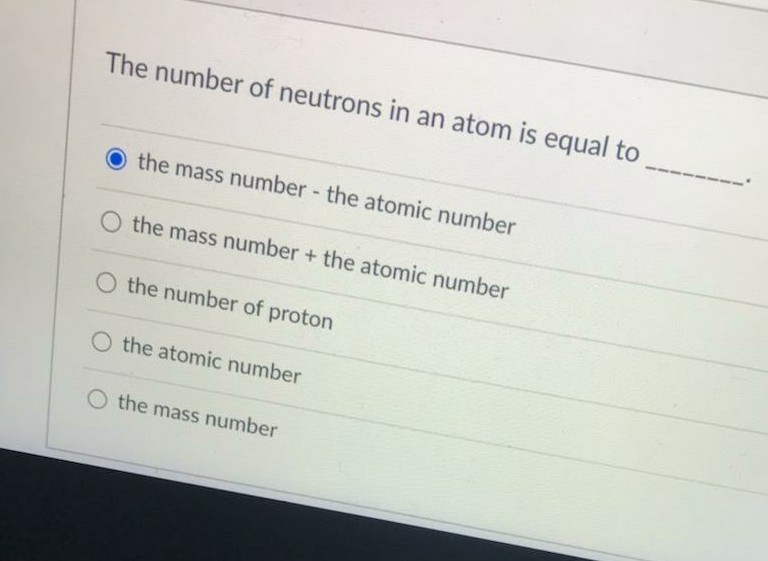
- The Number Of Neutrons In An Atom Is Equal To ..
- The Number Of Protons In An Atom Is Equal To Its Brainly
- See More Results
- The Number Of Protons In An Atom Is Equal To A Number Called The
The center of an atom that is formed by neutrons, protons, and electrons. A tiny particle that makes up all matter. A molecule that is formed from two or more different types atoms. A molecules that is formed from the same type of atoms. Tags: Question 8. Number of neutrons can be equal to or greater than the number of protons because mass number is equal to double the atomic number or greater than double the atomic number. Of course, number of neutrons can be greater than the number of electrons because number of electrons = number of protons in the neutral atom.
Why is the number of protons and electrons equal in an atom?
1 Answer
Actually the proton and electron count of an atom are equal only when the atom is neutral in charge.
The Number Of Neutrons In An Atom Is Equal To ..
The three atomic particles of an atom are the protons, which carry a positive charge, the electrons which carry a negative charge and the neutrons which have no charge. The proton number is always the same as the atomic number for the element.
The protons and neutrons are found in the nucleus of the of the atom and make up the major it of the mass of the atom. The electrons are found in orbitals surrounding the nucleus.
Free cross stitch software for mac. In order for the atom to remain electrically neutral the protons and electrons must balance each other. Free novel writing software for mac.
The Number Of Protons In An Atom Is Equal To Its Brainly
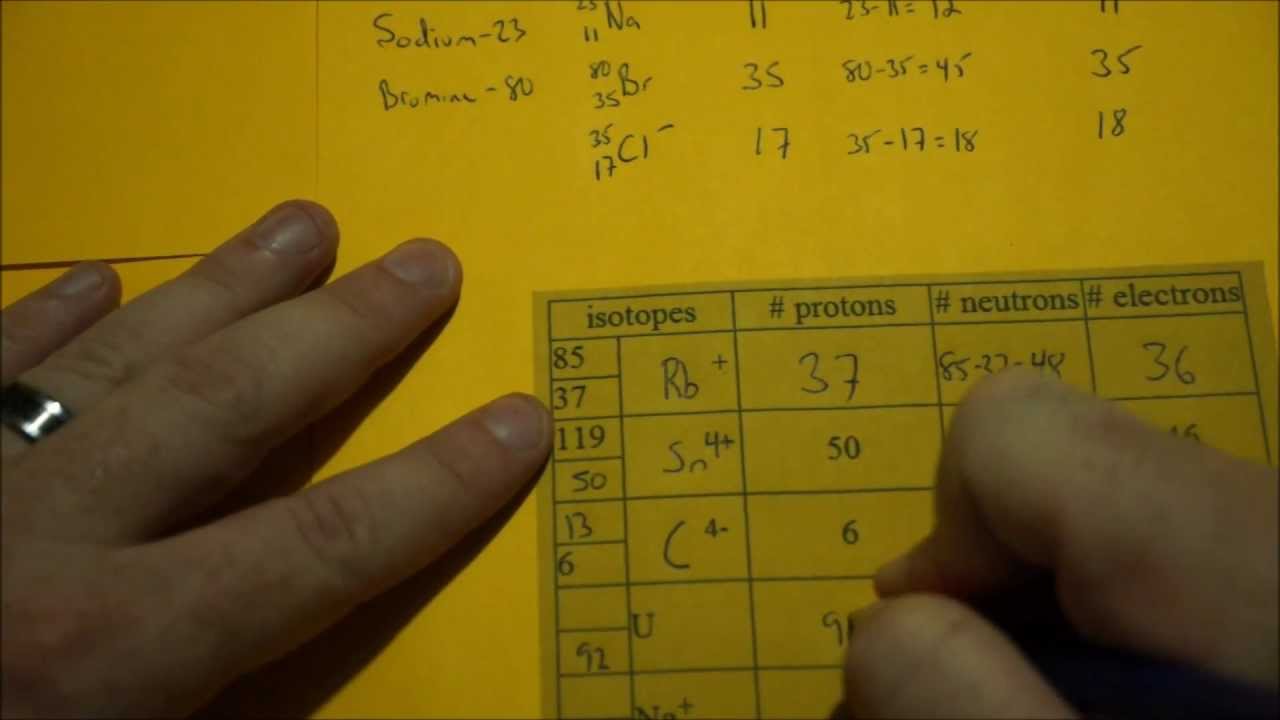
Download music from spotify mac free. Two examples would be Sodium (Na) and Chlorine (Cl)

Sodium has an atomic number of 11 and an amu of 23
Sodium has 11 protons 12 neutrons and 11 electrons.
11 protons - atomic number 11
11 + 12 = 23 the amu
Chlorine has an atomic number of 17 and an amu of 35
Sodium has 17 protons 18 neutrons and 17 electrons.
17 protons - atomic number 17
17 + 18 = 35 the amu
Related questions
The number of protons in an atom equals the number of what?
See More Results
1 Answer
The number protons equals the number of electrons in a NEUTRAL atom.
The Number Of Protons In An Atom Is Equal To A Number Called The
Explanation:
Protons are conceived to be positively charged, massive nuclear particles. Electrons are conceived to be particles of negligible mass that orbit the nuclear core. Clearly, for a neutral atom, the number of electrons must equal the number of nuclear protons. The number of nuclear protons,

Now an atom can gain or lose electrons to form a negatively or positively charged ion; it cannot lose nuclear protons because it that case the identity of the nucleus changes.
Related questions
